Introduction: Approved BCMA-directed cellular therapies (BDCT) for multiple myeloma (MM) include chimeric antigen receptor T cell therapies (CART): idecabtagene vicleucel (ide-cel), ciltacabtagene autoleucel (cilta-cel), and T cell engagers (TCE) such as teclistamab (tec). Despite promising efficacy and safety profiles, limited access is a challenge since the first BDCT approval in 2021. We conducted this TACTUM-23 survey to gain insights into the practices and perspectives of oncologists from BDCT-referring and treating centers and identify potential areas of improvement.
Methods: The cross-sectional survey was developed with input from BDCT-prescribing oncologists, referring oncologists, and a biostatistician. “ BDCT centers” were centers administering BDCT and included “CART-centers” and TCE-Centers“ where these respective therapies were administered, while others were grouped as ”BDCT-referring centers“ including ”CART/ TCE- referring centers“. Three sections were included: demographics, choice of BDCT in real-world scenarios, and logistical barriers to managing adverse effects at referring centers. The survey was administered electronically from 6/16/23-6/27/23 to a diverse sample of practicing US oncologists from various medical institutions collaborating with USMIRC. Only those who completed 100% of the survey by 7/1/23 were included in the interim analysis.
Results: The survey was distributed to 247 oncologists of which 66 (27%) responses were recorded. 37(15%) completed 100% of the survey and were included in this analysis. 73% of oncologists were from academic institutions, and 54% treated plasma cell disorders only. Most oncologists (70%) were from CART-centers. Similarly, most (81%) were from TCE-centers. 11% of centers offered only TCE and not CART while none exclusively offered CART alone. Among CART-centers, 54% offered both approved therapies (ide-cel/cilta-cel). Oncologists practiced in the Midwest (43%), South (22%), West (19%), and Northeast (16%).
Choice of BDCT in Real-World Scenarios: TCE was the favored BDCT for patients meeting criteria but with either significant comorbidity, renal failure, poor performance status, mild cognitive impairment, age >75yr, rapidly progressive disease, or post-CART relapse. CART was preferred only for scenarios of extramedullary disease, allogeneic stem cell transplant recipients, and renal failure on dialysis.
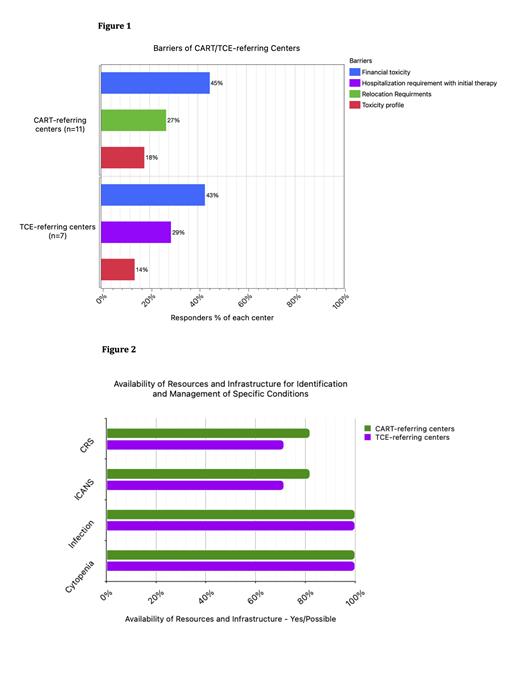
Respondents ranked choices of potential barriers to access based on their order of importance as below ( Figure 1)
Barriers to CART access: Financial burden was the top-ranking barrier (45%) by oncologists from CART-referring centers, while the number of production slots allocated per month was the top barrier (69%) by oncologists from CART-centers. Notably, relocation and caregiver requirements were ranked as top barriers by 27% of oncologists from CART-referring centers but by none of the oncologists from CART-centers.
Barriers to TCE access: Among oncologists from TCE-referring centers, the majority (43%) felt that financial burden was the top barrier to access for TCE while most oncologists (63%) from TCE-centers identified hospitalization requirements as the top barrier.
Infrastructure for side effect management in BDCT-referring centers: Most oncologists at BDCT-referring centers reported the ability to manage low-grade cytokine release syndrome (CRS), immune effector cell therapy associated neurotoxicity syndrome (ICANS), infections and cytopenia, as shown in Figure 2
Conclusions: While limited by the relatively low number of responses, this survey is the first to specifically delineate barriers to BDCT access by treating versus referring centers in MM. The primary concerns are financial burden, relocation requirements for CART, and hospitalization for TCE. Most oncologists at BDCT-referring centers expressed comfort with identifying and managing low-grade CRS and ICANS, suggesting the readiness of referring oncologists to engage in toxicity management. Future steps to increase collaboration between treating and referring centers include outpatient BDCT programs and clearer guidelines for transferring patients after the initial month of BDCT therapy. Relocation and caregiver requirements, which were perceived as barriers by referring but not treating centers, need to be addressed as well.
Disclosures
Hashmi:Karyopharm: Speakers Bureau; BMS: Honoraria; Jannsen: Honoraria, Speakers Bureau; Sanofi: Honoraria, Speakers Bureau; GSK: Honoraria, Speakers Bureau. McGuirk:Pluristem Therapeutics: Research Funding; Bellicum Pharmaceuticals: Research Funding; Astellas Pharma: Research Funding; Fresenius Biotech: Research Funding; Novartis: Research Funding; EcoR1 Capital: Consultancy; Magenta Therapeutics: Consultancy; Allovir: Consultancy, Research Funding; Juno Therapeutics: Consultancy; Kite: Consultancy, Research Funding; Gamida Cell: Research Funding. Banerjee:BMS: Consultancy; Caribou: Consultancy; Janssen: Consultancy; Genentech: Consultancy; Sanofi: Consultancy; SparkCures: Consultancy; Pfizer: Consultancy; Pack Health: Research Funding. Ahmed:BMS: Consultancy; Kite: Consultancy, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal